The Determination of Keqfor FeSCN2+
A
chemical system is claimed to be in balance when there are no quantifiable modifications happening. An instance of chemical balance is revealed listed below:
aA + bB <---> cC + dD
When catalysts An as well as B are initial blended, the system is not in stability. As the response earnings, the decline in the focus of the catalysts can be gauged in addition to a rise in the focus of the items. Since all responses are relatively easy to fix to some extent, as quickly as items C and also D are created, they respond backwards to develop items An as well as B once again as revealed listed below:
cC + dD <---> aA + bB
The price of a response usually differs with the focus of catalysts, i.e. lowering when the reactant focus reduce, as well as boosting when the reactant focus raise.
Ultimately, a problem of fixed focus or a stability state is gotten to. When the placement of the balance is much to the reactant side, it is claimed that there is no evident response. When the setting of the stability is much to the item side, it is claimed that the response goes almost to conclusion. For a system to be in balance implies that the focus of all chemical varieties do not alter with time as well as the temperature level, quantity, as well as stress stay continuous. Nevertheless, on a molecular range, stability is not fixed however a vibrant problem, where chemical modifications are still taking place however are not noticeable.
Both the forward as well as reverse responses remain to happen at stability. Due to the fact that their prices are equivalent, there is no internet modification in reactant focus. When balance is gotten to, it is kept just if all pertinent aspects stay the exact same. An adjustment in reactant focus, temperature level, stress, or quantity will certainly interrupt the stability creating the system to undertake extra web modifications to develop a brand-new balance.
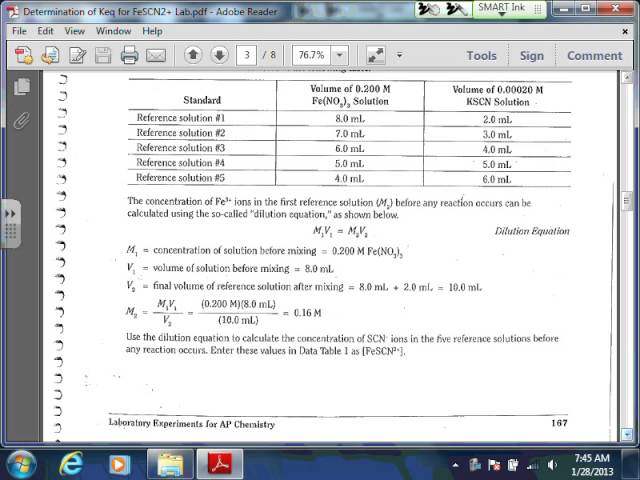
Partially A of this laboratory, a collection of recommendation services are prepared by responding big quantities of Fe3+ (iron( III) ions) with well-known quantities of SCN– (thiocyanate) ions. The examination services are prepared by blending continuous quantities of Fe3+ ions with various quantities of SCN– ions. The examination services consist of unidentified focus of FeSCN2+ ions at stability. Both services stand for the relatively easy to fix response revealed listed below:
Fe3+( aq) + SCN–( aq) <---> FeSCN2+( aq)
Using colorimetry, the absorbance worths of the recommendation options are identified as displayed in the information table listed below.
|
Experience
|
[FeSCN2+] (M)
|
Absorbance
|
|
Ref Solution 1
|
4.0 x 10-5
|
0.170
|
|
Ref Solution 2
|
6.0 x 10-5
|
0.255
|
|
Ref Solution 3
|
8.0 x 10-5
|
0.362
|
|
Ref Solution 4
|
1.0 x 10-4
|
0.430
|
|
Ref Solution 5
|
1.2 x 10-4
|
0.512
|
The example information over was made use of to generate the calibration contour for the absorbance of FeSCN2+. A calibration contour is made by outlining Absorbance (at a specific wavelength) vs [FeSCN2+] of the referral options.
 |
By making use of the absorbance analyses of the examination services, the calibration contour is made use of to identify the unidentified focus of FeSCN2+.
© 2004, Flinn Scientific, Inc. All Rights Reserved. Duplicated for single usage with consent from Flinn Scientific, Inc., Batavia, IL, USA. None of this product might be replicated or sent in any type of kind or whatsoever, digital or mechanical, consisting of, yet not restricted to xerox, videotaping, or any kind of info storage space as well as access system, without consent in creating from Flinn Scientific, Inc.; and also
That the Licensee consents to compensate and also hold Flinn Scientific, Inc. safe from any type of and also all obligation, loss, problems, prices of cost which Flinn Scientific, Inc. might hereafter sustain, experience or be needed to pay because such magazine by the Licensee; and also
To make up Flinn Scientific, Inc. $0.00 for the authorization to replicate this product.