Table of Contents
Including Solid Acid-Strong Base to Barrier
1
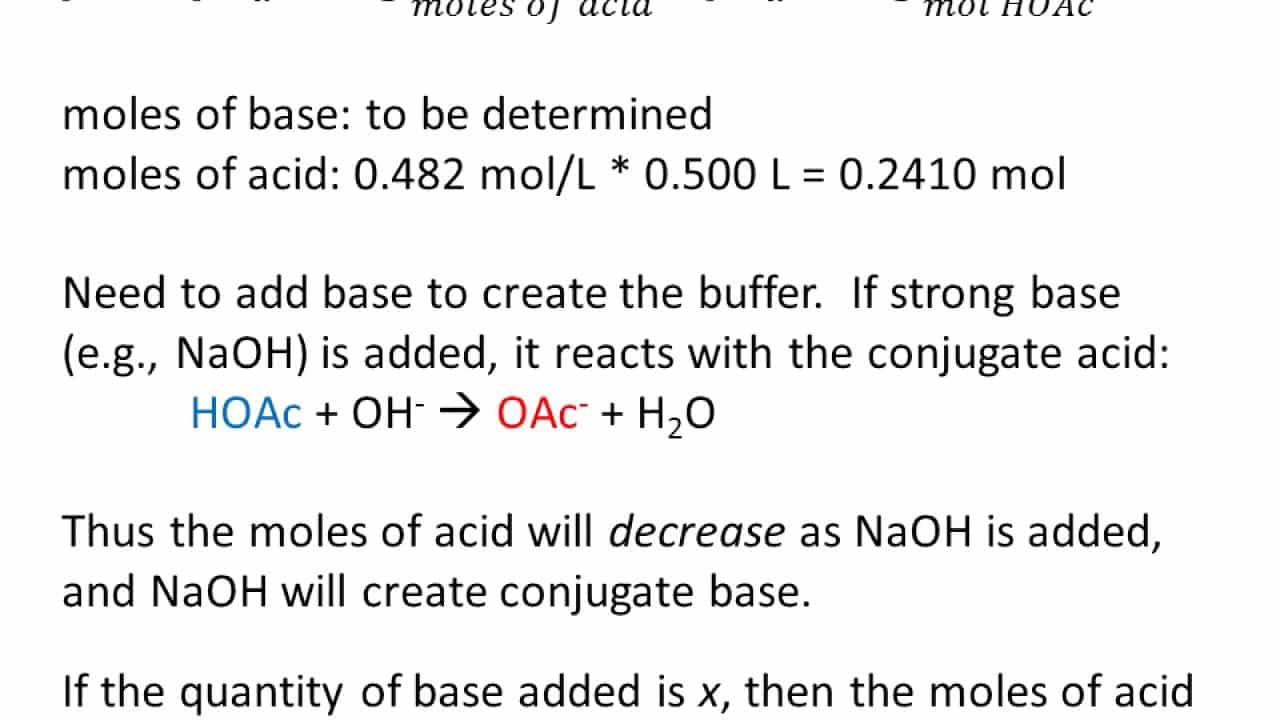
) What quantity of a 2.50 M HCl service need to be contributed to 500. mL of a 0.150 M CARBON MONOXIDE32- remedy to prepare a barrier with pH = 10.00?
(a) 20.29 mL
(b) 62.68 mL
(c) 9.71 mL
(d) 7.44 mL
2
) What quantity of a 3.00 M HCl service need to be included in 200. mL of a 0.200 M CARBON MONOXIDE32- remedy to prepare a barrier with pH = 10.30?
(a) 6.51 mL
(b) 6.82 mL
(c) 14.36 mL
(d) 13.96 mL
3
) What quantity of a 4.00 M HCl service should be contributed to 250. mL of a 0.250 M NH3 service to prepare a barrier with pH = 9.10?
(a) 22.59 mL
(b) 9.24 mL
(c) 6.39 mL
(d) 7.44 mL
4
) What quantity of 5.00 M salt hydroxide must be included in 1.00 L of a 0.200 M acetic acid option to prepare a barrier with pH = 4.50?
(a) 25.39 mL
(b) 14.61 mL
(c) 23.02 mL
(d) 17.63 mL
5
) What quantity of 4.50 M salt hydroxide have to be included in 250. mL of a 0.200 M acetic acid remedy to prepare a barrier with pH = 5.00?
(a) 20.22 mL
(b) 7.17 mL
(c) 19.02 mL
(d) 3.94 mL
6
) What quantity of 4.50 M salt hydroxide should be contributed to 250. mL of a 0.200 M nitrous acid service to make a barrier with pH = 3.00?
(a) 7.68 mL
(b) 4.96 mL
(c) 6.77 mL
(d) 3.43 mL
7
) What quantity of 3.50 M potassium hydroxide should be contributed to 500. mL of a 0.200 M carbonic acid remedy to prepare a barrier with pH = 6.30? (Assume just the very first hydrogen is ionized.)
(a) 13.55 mL
(b) 12.97 mL
(c) 15.60 mL
(d) 23.76 mL
House Online Assignments