Titration Curves
1
) The titration of a weak acid with a solid base is revealed listed below. What is the approxiamate Ka for the acid?
pH
vs Volume
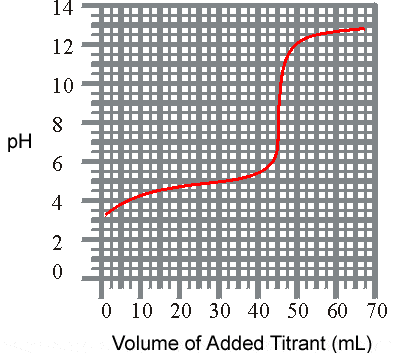
(a) 1.8 x 10-5
(b) 6.3 x 10-4
(c) 3.6 x 10-6
(
d) 3.2 x 10 -9 2)The titration of a weak base with a solid acid is revealed listed below. What is the approxiamate K b for the base?
pH
vs Volume
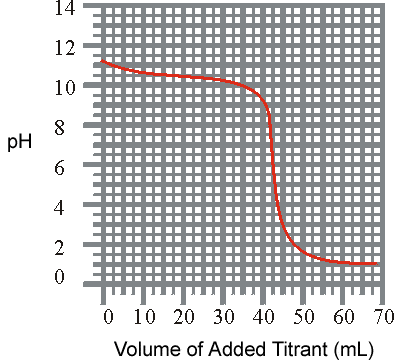
(a) 3.1 x 10-4(
b ) 3.2 x 10-11(c)1.0 x 10 -8(d)
1.0 x 10-6
3
)The titration of a weak acid with a solid base is revealed listed below. What is the approxiamate Ka for the acid?
pH
vs Volume
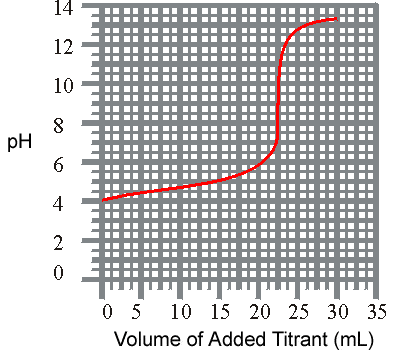
(a) 8.8 x 10-2(b
) 1.0 x 10-4
( c) 1.6 x 10-9(d
)
1.6 x 10 -5 4 )The titration of a weak base with a solid acid is revealed listed below. What is the approxiamate Kb for the base?
pH
vs Volume
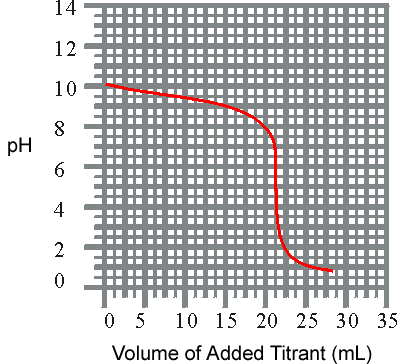
(a)
7.9 x 10-11(b
) 2.5 x 10-5(c
) 1.3 x 10-4 (d) 4.0 x 10 -10 Residence Online Assignments


















