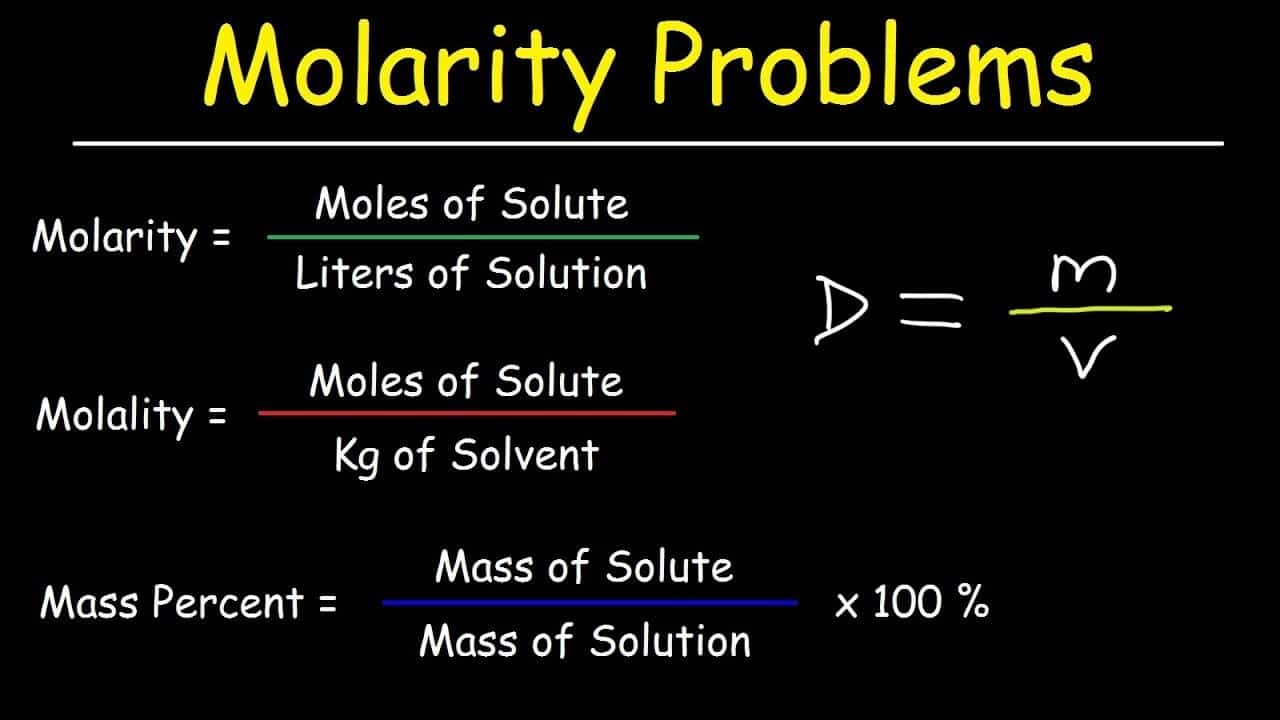
Molality
1
) What mass of salt chloride is needed to decrease the cold factor of 6.50 kg of water to -31.7 C?
( a) 2.55 kg
( b) 3.24 kg
( c) 5.68 kg
( d) 6.47 kg
( e) 8.52 kg
2
) What mass of bromine requires to be contributed to 950. ml of carbon tetrachloride to make a 0.25 m service? The thickness of Br2 is 3.12 g/mL which of CCl4 is 1.59 g/mL.
( a) 38 g
( b) 60. g
( c) 40. g
( d) 64 g
3
) What mass of lithium sulfate is called for to reduce the cold factor of 7.20 kg of water to -31.7 C?
( a) 4.50 kg
( b) 5.68 kg
( c) 3.55 kg
( d) 6.75 kg
( e) 13.5 kg
.
4) What mass of high levels of caffeine requires to be included in 450. ml of acetone to make a 0.85 m option? The thickness of high levels of caffeine is 1.23 g/mL, the molecular mass of high levels of caffeine is 194.20 g, the thickness of acetone is 0.790 g/mL and also the molecular mass of acetone is 58.08 g.
( a) 170 g
( b) 74 g
( c) 130 g
( d) 59 g
.
5) What mass of trimethylene glycol, HOCH2CH2CH2OH, is called for to decrease the cold factor of 7.50 kg of water to -27.8 C?
( a) 4.26 kg
( b) 14.9 kg
( c) 2.84 kg
( d) 8.52 kg
( e) 5.52 kg
.
6) What mass of citric acid requires to be contributed to 950. ml of acetone to make a 0.30 m remedy? The thickness of citric acid is 1.67 g/mL, the molecular mass of citric acid is 192.10 g, the thickness of acetone is 0.790 g/mL and also the molecular mass of acetone is 58.08 g.
( a) 55 g
( b) 58 g
( c) 46 g
( d) 43 g
.