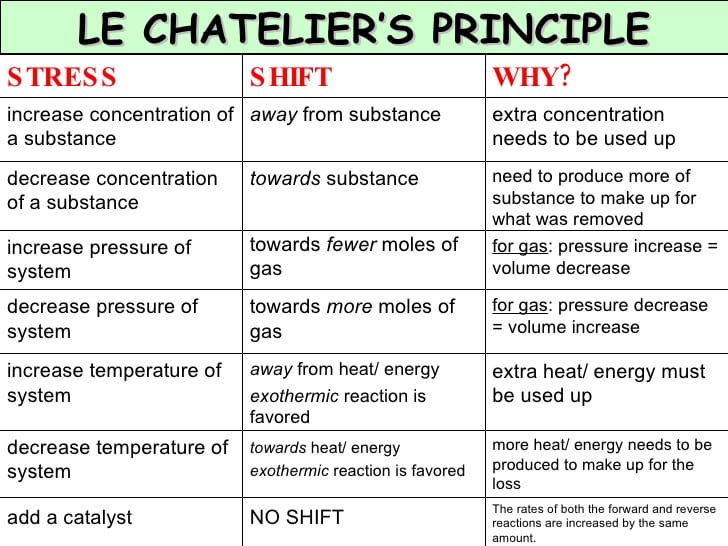
Le Chatelier’s Concept
1
) Consider the adhering to stability, developed in a 2-L flask at 30 C.
CARBON MONOXIDE( g) + H2O( g) ![]() < img elevation=" 12" src=” https://mmsphyschem.com/wp-content/uploads/2017/08/eqarrow-1240042.gif” size =” 39 “> CARBON MONOXIDE 2( g) +H 2 (g) ΔH °= -41.2 kJ What will occur to the focus of carbon monoxide if the temperature level is enhanced? (a) It will certainly boost. (b) It will certainly reduce.( c) It will certainly not transform.
< img elevation=" 12" src=” https://mmsphyschem.com/wp-content/uploads/2017/08/eqarrow-1240042.gif” size =” 39 “> CARBON MONOXIDE 2( g) +H 2 (g) ΔH °= -41.2 kJ What will occur to the focus of carbon monoxide if the temperature level is enhanced? (a) It will certainly boost. (b) It will certainly reduce.( c) It will certainly not transform.
2) Consider the adhering to stability
, developed in a 3-L flask at 1227 C.
2SO 2( g)+ O 2( g)< img elevation=" 12" src=" https://mmsphyschem.com/wp-content/uploads/2017/08/eqarrow-1240042.gif" size=" 39" >
2SO 3( g) K= 0.15 What will certainly occur to the focus of SO 3 if the focus of
SO2is enhanced?( a) ![]() It will certainly enhance.( b) It will certainly reduce.
It will certainly enhance.( b) It will certainly reduce.
( c) It will certainly not transform. 3) Consider the complying with stability, developed in a 3-L flask at50 C. NiO (s )+ CARBON MONOXIDE( g)< img elevation=
” 12 “src=” https://mmsphyschem.com/wp-content/uploads/2017/08/eqarrow-1240042.gif” size =” 39″ > Ni( s)+ CARBON MONOXIDE 2 (g) Which instructions
will the stability change if the stress is enhanced?( a) To the right. (b) To the left. ( c) The placement of the stability will certainly not alter. 4) Consider the adhering to stability, developed in a 1-L flask at 20 C. Fe 3 O4( s) + CARBON MONOXIDE( g )< img elevation=" 12 "src =" https://mmsphyschem.com/wp-content/uploads/2017/08/eqarrow-1240042.gif" size=" 39" > 3FeO (s )+ CARBON MONOXIDE 2( g) ΔH °= 19 kJ What will occur to the focus of carbon monoxide if the temperature level is lowered?( a ) It will certainly enhance.( b) It will certainly lower.( c) It will certainly not transform. 5)Considerthe adhering to balance, developed in a 1-L flask at 20 C. Fe 3 O 4( s)+ CARBON MONOXIDE( g)< img elevation=" 12" src=" https://mmsphyschem.com/wp-content/uploads/2017/08/eqarrow-1240042.gif " size="39 "> 3FeO (s)+ CARBON MONOXIDE 2( g) ΔH °= 19 kJ What will certainly take place to the focus of carbon monoxide 2 if even more Fe 3 O 4 is included ?( a) It will certainly enhance. ( b) It will certainly lower. ( c ) It will certainly not alter. 6) Consider the complying with balance, developed in a 2-L flask at 50 C. CS 2 ( g)+4H 2( g) instructions will the stability change ?( a) To the right. ( b ) To the left.( c) The setting of the balance will certainly not transform. 7) Consider the complying with stability, developed in a 1-L flask at 20 C. CARBON MONOXIDE( g)+ H 2 O( g)< img elevation=" 12" src=" https://mmsphyschem.com/wp-content/uploads/2017/08/eqarrow-1240042.gif" size= “39” > CARBON MONOXIDE 2 (g )+ H 2( g) ΔH ° =-41.2 kJ What will certainly occur to the focus of carbon monoxide 2 if the temperature level is lowered? (a) It will certainly raise.( b) It will certainly lower.( c) It will certainly not alter. 8 ) Consider the complying with stability, developed in a 2-L flask at 40 C. CH 4( g) +2H 2 S( g) ( a) To the right. ( b) To the left. ( c) The setting of the stability will certainly
not alter .. Residence Online Assignments
![]() < img elevation=" 12" src="https://mmsphyschem.com/wp-content/uploads/2017/08/eqarrow-1240042.gif" size=" 39" > CH 4( g)+ 2H 2 S( g )If the stress is raised, in which
< img elevation=" 12" src="https://mmsphyschem.com/wp-content/uploads/2017/08/eqarrow-1240042.gif" size=" 39" > CH 4( g)+ 2H 2 S( g )If the stress is raised, in which
![]() < img elevation=" 12" src=” https://mmsphyschem.com/wp-content/uploads/2017/08/eqarrow-1240042.gif” size =” 39 “> CS 2( g) +4H 2( g) If the stress isreduced, in which instructions will the balance change?
< img elevation=" 12" src=” https://mmsphyschem.com/wp-content/uploads/2017/08/eqarrow-1240042.gif” size =” 39 “> CS 2( g) +4H 2( g) If the stress isreduced, in which instructions will the balance change?