Table of Contents
Intermolecular Pressures
1
) Which of the adhering to declarations regarding intermolecular pressures is inaccurate!.
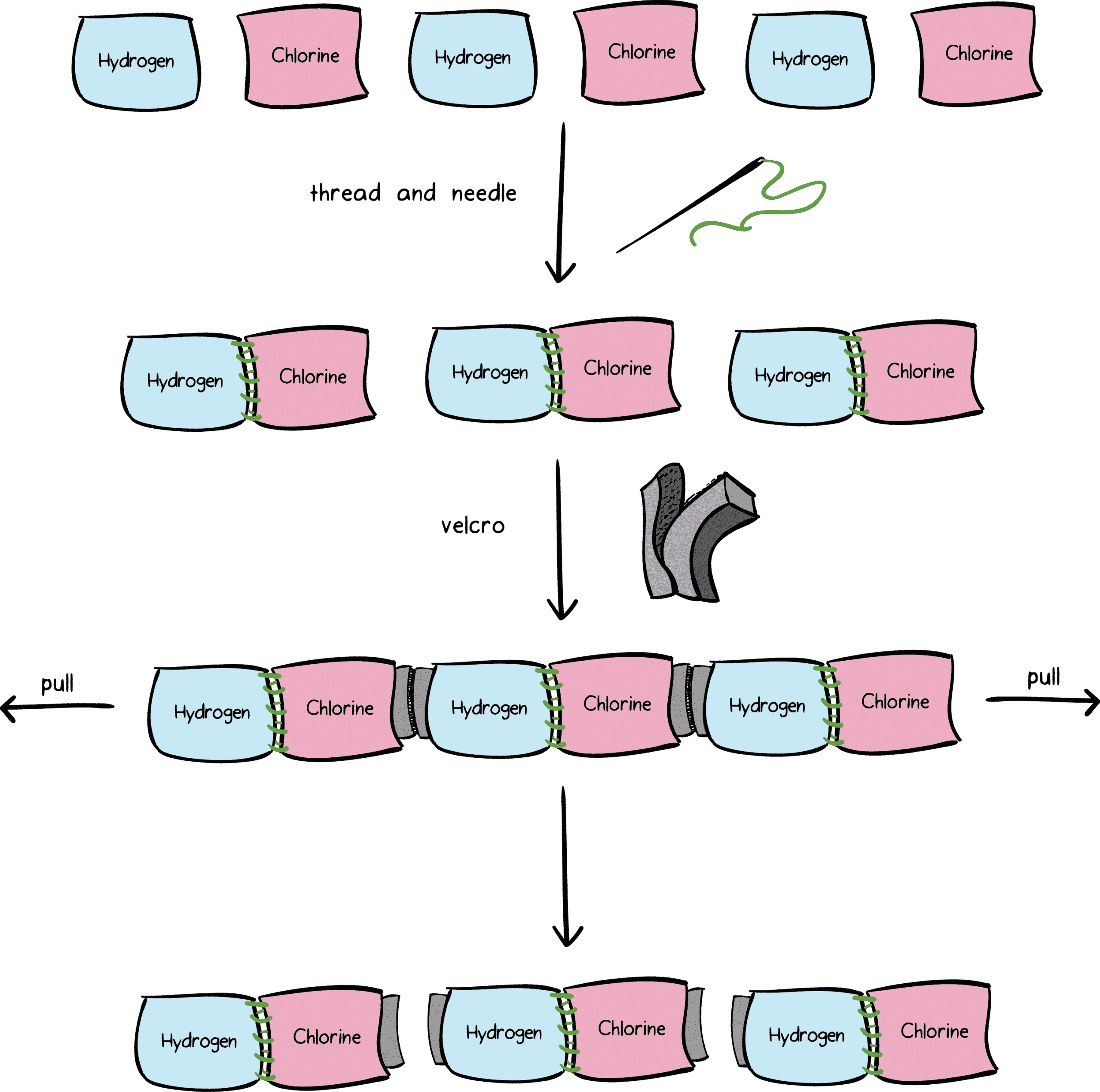
?.!?( a) The only intermolecular pressures in between nonpolar particles are London pressures.
( b) Dipole-dipole destinations happen in between polar particles.
( c) Hydrogen bonds are an unique type of dipole-dipole destination.
( d) The visibility of hydrogen bonding make up most of the one-of-a-kind residential properties of water.
( e) Intermolecular pressures establish the shade of molecular materials.
2
) Which of the complying with declarations concerning intermolecular pressures is wrong!.
?.!?( a) Intermolecular pressures define the tourist attractions in between 2 or even more different particles
.( b )The only intermolecular pressures in between nonpolar particles are London pressures.
( c) The existence of hydrogen bonding make up a lot of the special homes of water.(
d) Dipole-dipole tourist attractions happen in between polar bonds.
( e) Hydrogen bonds are an unique type of dipole-dipole destination.
3
) Which of the adhering to declarations regarding intermolecular pressures is inaccurate!.
?.!?( a) Intermolecular pressures define the destinations in between 2 or even more different particles
.( b )The only intermolecular pressures in between nonpolar particles are London pressures.
( c) Dipole-dipole destinations are the greatest type of intermolecular pressure.
( d) Intermolecular pressures represent several homes of molecular compounds such as melting factor, boiling factor, and so on(
e) Dipole-dipole tourist attractions take place in between polar particles.
4
) Which of the adhering to declarations concerning intermolecular pressures is inaccurate!.
?.!?( a) Intermolecular pressures define the tourist attractions in between 2 or even more different particles
.( b )The only intermolecular pressures in between nonpolar particles are London pressures.
( c) Intermolecular pressures just depend upon the polarity of the particles.
( d) Hydrogen bonds are an unique sort of dipole-dipole communication.
( e) The visibility of hydrogen bonding make up a lot of the special homes of water.
5
) Which of the adhering to declarations concerning intermolecular pressures is inaccurate!.
?.!?( a) Intermolecular pressures explain the destinations in between 2 or even more different particles
.( b )Intermolecular pressures represent numerous residential or commercial properties of molecular materials such as melting factors, steaming factors, and so on(
c) Nonpolar particles with polar bonds can have dipole-dipole destinations as well as London pressures.
( d) Hydrogen bonds are an unique sort of dipole-dipole destination.
( e) The visibility of hydrogen bonding make up most of the special homes of water.
6
) Which of the complying with declarations regarding intermolecular pressures is inaccurate!.
?.!?( a) Intermolecular pressures define the tourist attractions in between 2 or even more different particles
.( b )The only intermolecular pressures in between nonpolar particles are London pressures.
( c) Dipole-dipole destinations happen in between polar particles.
( d) Hydrogen bonds are an unique sort of covalent bond.
( e) The visibility of hydrogen bonding make up a number of the distinct residential or commercial properties of water.
7
) Which of the complying with declarations regarding intermolecular pressures is wrong!.
?.!?( a) Intermolecular pressures define the pressures holding the atoms with each other in a particle.
( b) Intermolecular pressures make up numerous homes of molecular compounds such as melting factors, steaming factors, and so on(
c) The only intermolecular pressures in between nonpolar particles are London pressures.
( d) Dipole-dipole destinations take place in between polar particles.
( e) Hydrogen bonds are an unique sort of dipole-dipole tourist attraction.
8
) Which of the complying with declarations concerning intermolecular pressures is wrong!.
?.!?( a) Intermolecular pressures explain the destinations in between 2 or even more different particles.
( b) Intermolecular pressures make up several homes of molecular compounds such as melting factors, steaming factors, and so on(
c) The only intermolecular pressures in between nonpolar particles are London pressures.
( d) Dipole-dipole destinations happen in between polar particles.
( e) Hydrogen bonds happen in between hydrogen and also any type of various other component.
.