Density And Error Analysis
< img align= "right" elevation=" 192 "src=" https://mmsphyschem.com/wp-content/uploads/2018/03/thermo-4018364.jpg" size =" 127" > Density is an action of the amount of mass of a compound each of quantity. Due to the fact that thickness can be gauged without a chemical modification happening, it is a physical residential property. Thickness is likewise an extensive home due to the fact that it does not rely on the quantity important being determined. Because of this, thickness can be made use of to assist recognize an unidentified compound.
Mathematically, thickness is revealed as D = M/V and also has devices of g/mL or g/cm3.
From the above formula, it appears that thickness is both temperature level as well as stress reliant. For fluids as well as solids, the thickness will certainly alter somewhat with adjustments in temperature level and also stress. Many solids and also fluids broaden when they are heated up which boosts their quantity. The noteworthy exemption is water when it is warmed from 0 ° C to 4 ° C since it agreements. Overlooking any type of small dissipation, home heating cause a reduction in thickness. Modifications in stress can raise or lower the quantity however the resulting adjustment in thickness is minimal. Unless mentioned, the thickness of solids and also fluids are identified at space temperature level, 25 ° C.
The thickness of gases differs considerably relying on the temperature level and also stress. To figure out the thickness of a gas at STP (Standard Temperature And Pressure), the quantity needs to be gauged at STP. Requirement temperature level and also stress is 0 ° C (273 K) and also 1 atm machine (101.3 kPa, 760 mm).
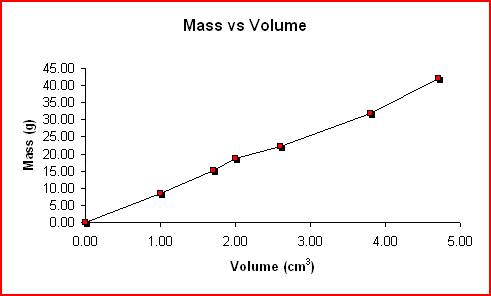
The complying with mass as well as quantity information was accumulated by utilizing the water variation technique for 6 various items of copper. The thickness was computed for every test and also the chart of Mass vs Volume is revealed listed below.
< img
elevation=” 296″ src=” https://mmsphyschem.com/wp-content/uploads/2018/03/mvsvol-7147685.gif” size=” 491″ > Mass Quantity Thickness( One technique
of
reporting
mistakes
in
dimension is by mentioning the precision
of
the dimensions. Precision describes the
nearness of a dimension to the approved worth of the dimension. Percent mistake identifies the precision of a collection of dimensions and also is established by the adhering to expression: %mistake =| O-|/ A x 100 %where O is the observed worth and also A is the approved worth. The approved worth for the thickness of copper is 8.92 g/cm 3 as well as
the standard of the thickness determined
over is 8.78 g/cm 3. Replacing these worths right into the expression over returns: %mistake=|8.78 g/cm 3- 8.92 g/cm 3 |/ 8.92 g/cm3 x 100%= 1.6% Another technique of reporting mistakes is by mentioning the accuracy
of the information. Accuracy describes just how close the gauged worthsare to each other. The typical or mean worth of a collection of information is determined
by separating the amount of the information by the variety of information worths. standard( mean )worth, D ave =( D 1+ D 2+ D 3+ D 4+ D 5+ D 6)/ 6 D ave =( 8.60+ 8.94+ 9.30+ 8.50+ 8.39 +8.98 )g/cm 3/ 6 =8.78 g/cm 3 The chart of Number Of Occurences vs Density revealed listed below create a mistake contour for reduced accuracy information. When mistakes are big leading to reduced accuracy information, theinformation are extra expanded and also thecontour is wider as well as much less sharp.< img
elevation=”295″ src=” https://mmsphyschem.com/wp-content/uploads/2018/03/trend-2489316.gif” size=” 491 “> For high accuracy information,
the majority of worths will certainly be gathered around the standard (although some will certainly exist additionally away) as well as the contour is narrower and also sharper. An additional technique of mentioning the accuracy is determining the conventional variance. Typical inconsistency is a procedure of the size of the
mistake contour and also suggests the accuracy of the information. To compute the common inconsistency
,
s, of the thickness: Calculate the standard or indicate thickness, D ave. Deduct the mean from each thickness worth.
Square each of the distinctions as well as locate the amount of the distinctions. Split the amount of the squares by the variety of thickness worths, minus one( N-1). Take the square origin
of the cause the previous action. Using these actions
- to the determined thickness as well as utilizing Dave=
- 8.78 g/cm 3 generate the adhering to worths: D- D ave( D- D
- ave) 2( g/cm 3) (g/cm 3) 2 -0.18 0.032 0.16 0.026 0.52 0.27 -0.28 0.078 -0.39 0.15 0.20 0.040
- The formula for common discrepancy is offered by: s= √ (( Σ (D- D ave) 2)/( N-1
- )) Substituting the table values return: s =√)
( 0.032 2 +0.026 2 +0.27 2 +0.078 2 +0.15 2 +0.040 2+/ 5) =0.345 g/cm 3The smaller sized the basic variance, s, the closer the thickness
>
The typical inconsistency
for the
thickness
information is
0.345 g/cm
3. Basic variance constantly has the
very same devices as the initial information. A typical variance of 0.345
g/cm 3 ways that 68 %of succeeding thickness dimensions will certainly exist within ± 0.346 g/cm 3of the typical worth( ± 1 basic discrepancy). Theworths will certainly hinge on thearray:8.43 g/cm 3 ≤ 8.78g/cm 3 ≤ 9.13
g/cm 3 It likewise indicates that 95% of additional thickness worths will certainly exist within ± 2( 0.346 g/cm 3) or 0.652 g/cm 3 of the ordinary worth( ± 2 conventional discrepancies ). The worths will certainly depend on the array: 8.09 g/cm 3 ≤ 8.78 g/cm 3 ≤ 9.47 g/cm 3 Determining the basic inconsistency for bigger examples of information often tend to be tiresome and also mistake susceptible so it is extra reliable to make use of Microsoft Excel or a TI graphing
calculator with analytical capacities. One more technique of reporting mistakes is regressionevaluation. Regression evaluation is locating the straight line or smooth contour that ideal fits the information. The chart does not need to travel through all the factors neither does it need to travel through any one of the factors. Preferably,the chart must pass as carefully as feasible to every one of them with the exception of the outliers. The
formula for a straight connection in between x as well as y is: y= mx +b where m is
the incline and also b is the y-intercept. The incline of the line, which is typically of best rate of interest, is figured out by the expression: m= Δy/ Δx= y 2 – y 1/ x 2- x 1 When making use of a TI graphing calculator and/or Microsoft Excel there is somewhat various terms. The TI calls looking for the
line of finest healthy regression as well as Excel calls it a trendline. When making use of earlier TI’s, TI-82
, or Excel, the relationship variable, r, is likewise reported. The connection aspect, r, is constantly in between -1 as well as +1. The closer the r worth is to +1 or -1, the even more of a straight partnership
that exists in between x as well as y. It is typically concurred that an appropriate variety for r is: 0.6 ≤ r ≤ -0.6 The connection aspect is a dimensionless (unitless )worth. The direct regression or trendline for the Mass vs Volume worths is revealed listed below.< img elevation= "297" src=" https://mmsphyschem.com/wp-content/uploads/2018/03/mvsvolt-3560864.gif" size=" 492" > Many times, among the determined worths will certainly show up to substantially vary from the others. Intend the last worth
in the information table was 9.91 g/cm 3 as revealed listed below.
Mass Quantity Thickness (g)( centimeters 3)( g/cm 3 )0.00 0.00 0.00 8.60 1.00 8.60 15.20 1.70 8.94 18.60 2.00 9.30 22.10 2.60 8.50 31.90 3.80 8.39 45.60 4.60 9.91 The 2nd 5 worths remain in arrangement yet thelast worth appears extremely various
. Prior to throwing out the last worth, a Q-Test needs to be put on one of the most deviant worth. The Q-Test is offered by: Q =| deviant outcome- closest next-door neighbor |/ series of determined worths One of the most deviant thickness worth is 9.91 g/cm 3 as well as the closest thickness worth is 9.30 g/cm 3. The series of the information is the distinction in between the biggest thickness 9.91 g/cm 3 as well as the tiniest thickness 8.39 g/cm 3. Q is computed to be: Q=( 9.91 g/cm 3- 9.30 g/cm 3 )/( 9.91 g/cm 3 -8.39 g/cm 3)= 0.40 The Q worth of 0.40 is contrasted to a listing of common
Q worths revealed listed below. The
shortened table of typical Q-90 worths shows when a deviant worth might be turned down with 90% self-confidence that the deviant outcome is in mistake. Variety of Trials Q-90 2–.
3
.
0.94. 4. 0.76. 5. 0.64. 6. 0.56. 7. 0.51. 8. 0.47. 9. 0.44. 10. 0.41. If the Q worth is bigger than the table worth of Q, the deviant outcome needs to be disposed of prior to any type of estimations of the
mean or basic variance are done. In this instance,