Table of Contents
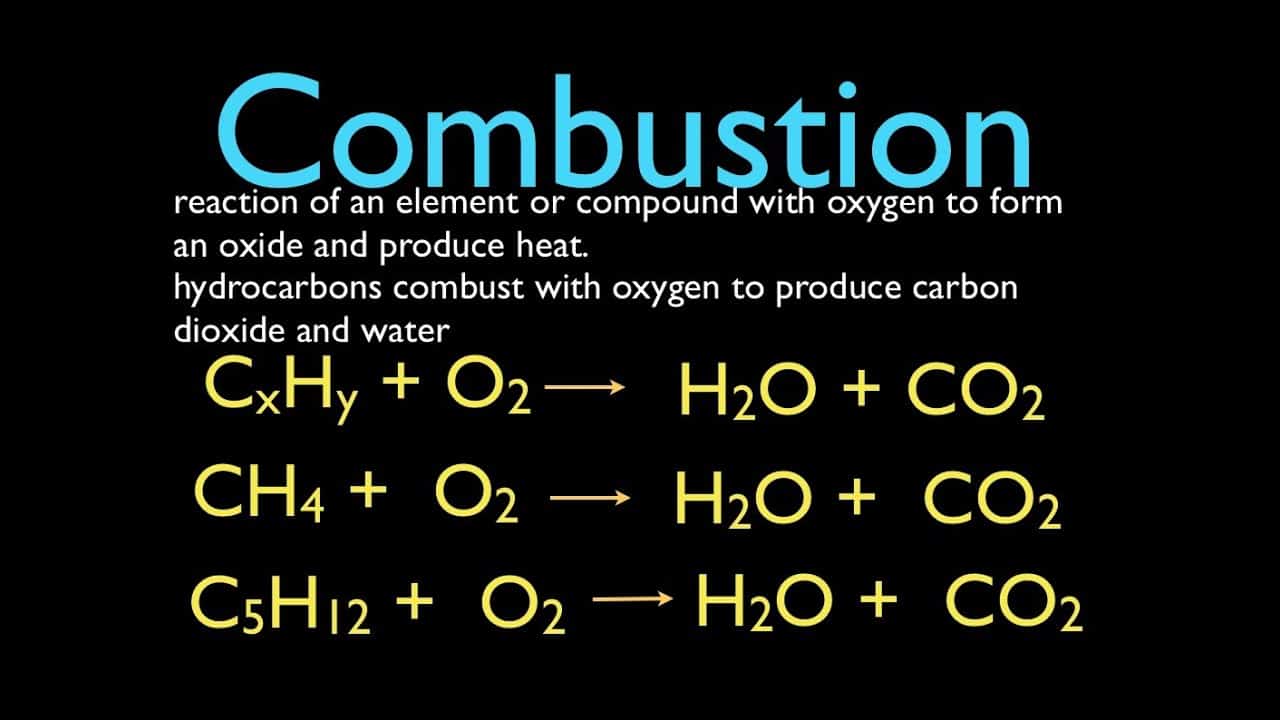
Burning Responses
1
) A 65.7 g example when shed generates 182 g CARBON MONOXIDE2 and also 51.7 g H2O. What is the formula of this substance? (The substance includes C, H, and also O.)
(a) C13H18O
(b) C7H9O
(c) C13H18O2
(d) C3H4O8
(e) C10H7O2
2
) A 68.2 g example when shed creates 150. g CARBON MONOXIDE2 and also 27.3 g H2O. What is the formula of this substance? (The substance contains C, H, as well as O.)
(a) C2H2O
(b) C9H8O4
(c) C9H8O22
(d) C9H4O4
(e) C9H8O2
3
) A 48.6 g example when melted generates 131 g CARBON MONOXIDE2 as well as 38.9 g H2O. What is the formula of this substance? (The substance contains C, H, and also O.)
(a) C11H16O
(b) C6H8O
(c) C11H16O2
(d) C4H6O11
(e) C11H8O2
4
) A 76.8 g example when melted creates 139 g CARBON MONOXIDE2 and also 24.4 g H2O. What is the formula of this substance? (The substance contains C, H, and also O.)
(a) C7H6O17
(b) C7H3O5
(c) C14H12O5
(d) CHO
(e) C7H6O5
5
) A 24.6 g example when melted generates 53.7 g CARBON MONOXIDE2 as well as 8.45 g H2O. What is the formula of this substance? (The substance includes C, H, and also O.)
(a) C13H5O6
(b) C13H10O3
(c) C2H2O
(d) C13H10O6
(e) C4H3O9
6
) A 43.5 g example when shed creates 105 g CARBON MONOXIDE2 and also 30.1 g H2O. What is the formula of this substance? (The substance contains C, H, and also O.)
(a) C10H14O3
(b) C10H14O27
(c) C10H7O3
(d) C20H28O3
(e) C3H5O
7
) A 26.7 g example when melted creates 69.8 g CARBON MONOXIDE2 and also 11.9 g H2O. What is the formula of this substance? (The substance contains C, H, as well as O.)
(a) C4H3O
(b) C12H10O3
(c) C12H10O29
(d) C12H5O3
(e) C24H20O3
8
) A 66.5 g example when shed creates 171 g CARBON MONOXIDE2 as well as 25.5 g H2O. What is the formula of this substance? (The substance contains C, H, as well as O.)
(a) C4H3O
(b) C11H8O3
(c) C4H3O10
(d) C11H4O3
(e) C22H16O3
9
) A 42.6 g example when shed generates 122 g CARBON MONOXIDE2 as well as 44.8 g H2O. What is the formula of this substance? (The substance contains C, H, and also O.)
(a) C20H36O2
(b) C5H18O2
(c) C10H18O
(d) CH2O3
(e) C10H9O
10
) A 83.5 g example when melted generates 169.8 g CARBON MONOXIDE2 and also 31.6 g H2O. What is the formula of this substance? (The substance contains C, H, as well as O.)
(a) C11H10O6
(b) C11H10O27
(c) C11H5O6
(d) C11H10O3
(e) C2H2O
Residence Online Assignments