Table of Contents
How to Calculate Bond Dissociation Energy
To calculate bond dissociation energy, you have to know the formula for a diatomic molecule. It contains two atoms, which can be the same or different. The bond dissociation enthalpy is the amount of energy required to break one mole of a chemical bond. This is a gaseous quantity, because it is impossible to calculate it in the solid or liquid states. Hydrogen chloride requires 432 kJ of energy to break into its two individual molecules. In order to calculate it, you need to know the formula for a hydrogen chloride molecule.

In order to calculate bond dissociation energy, you need to know the heat of molecule formation. This energy is obtained by dividing the atoms of a molecule by their dissociation energies. The heat of formation is the difference between the amount of energy required to break a single atom and the total energy necessary to break it. In other words, if a molecule is broken, the enthalpy is positive. If it is broken, then the dissociation will take place at the same time, while if it isn’t, then the bond will break in two. In this way, you can find out how to calculate bond dissociation energies.
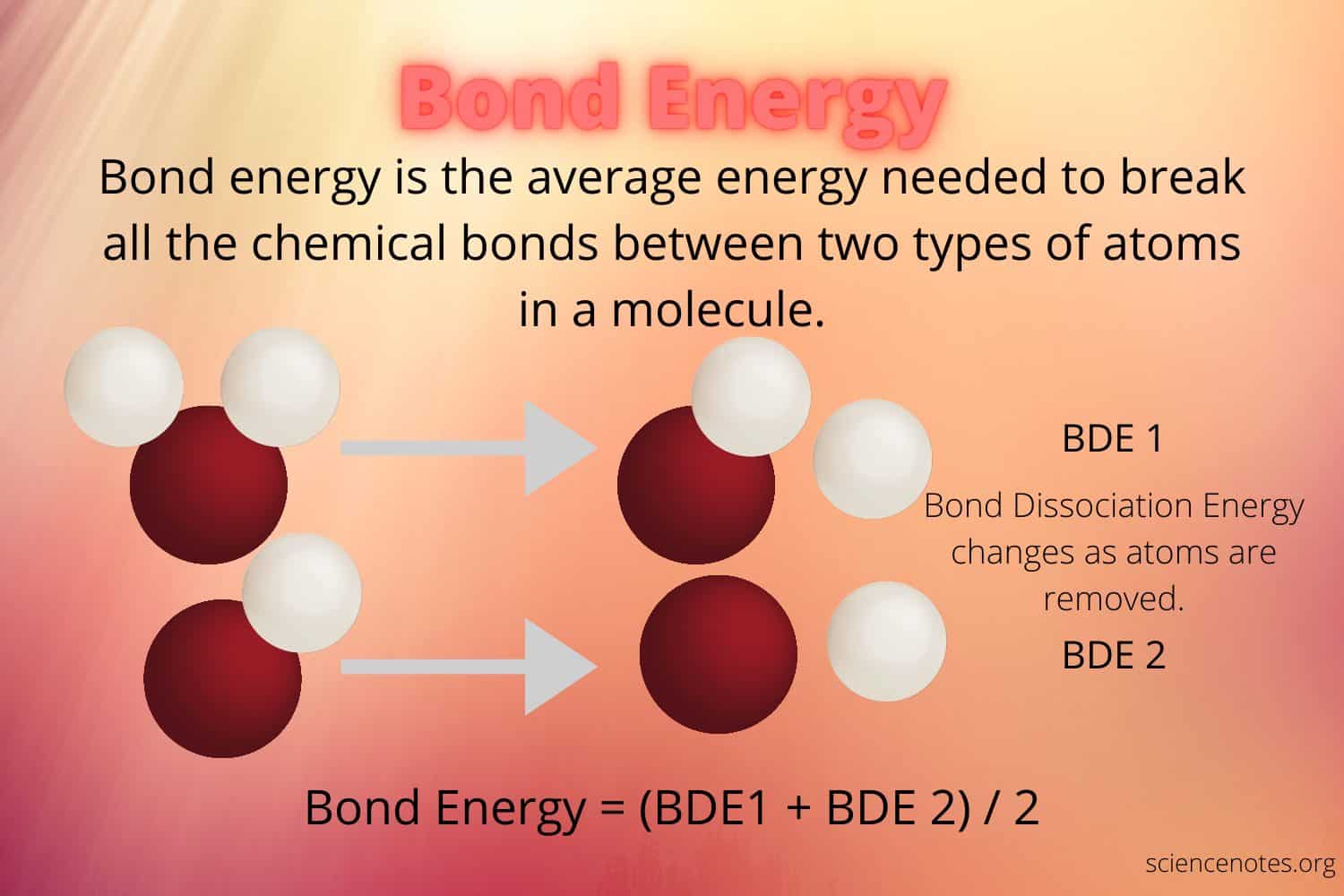
You can also calculate bond dissociation energy for a specific molecule. A dissociation energy is an average of the bond energies of the two different types of bonds in a given molecule. A negative value means the bond will dissolve without breaking. A negative value indicates that a single atom is unstable. If the atoms are not completely stable, they may react with one another in different ways, causing the bond to remain intact.
The energy of a single atom is not the same as the bond energy of a chemical compound. It refers to the energy needed to break a single atom’s bond. A bond between two atoms will generate a bond that is weaker than the molecule’s other bonds. A reaction between a single atom has a bond that has less energy. A chemical reaction will have a higher total energy than a reaction involving only one molecule.
The bond dissociation energy of a molecule is measured in kJ/mol. The IUPAC Gold Book does not prescribe a particular temperature for the bond-dissociation energy, but it provides a good idea of how much energy a chemical molecule can release. Its enthalpy is a measure of how many molecules are present in a molecule. Its standard enthalpy is the difference between the energy of the two compounds, and the corresponding amount of heat required to break each bond.
The bond dissociation energy of a molecule is an average of the bond dissociation energies of the different elements in the molecule. The enthalpy of a single molecule is 120 kJ/mol. In contrast, a molecule with a diatomic structure has a bond-dissociation energy of 101 kJ/mol. A molecule with a residual portion has an average of 110.5 kJ/mol.
Similarly, the bond dissociation energy of a molecule is a measure of how much energy is required to break a single atom. The DHdeg, or dissolved hydrogen, is the standard enthalpy of a reaction. Despite the fact that the energy of a single atom is zero, the bond energy of an atom is not. However, the enthalpy of a molecule is a positive number.
The bond dissociation energy is a state function of the bond between two atoms. The energy is not affected by the path of the bonds. The calculation is based on the average of the bond energies in several species. For example, an atom’s enthalpy will depend on the number of dissolved ions in the solution, and a molecule’s enthalpy will depend upon the number of ions in the solvent.
The bond dissociation energy of a given element depends on the atoms involved. For example, the oxygen hydrogen bond in water has a 120 kcal energy, while the second hydrogen atom in the molecule has a 101 kcal energy. The energies of two elements can also be affected by the residual part of the molecule. It is crucial to understand the bonds in a chemical system to ensure that it operates efficiently.