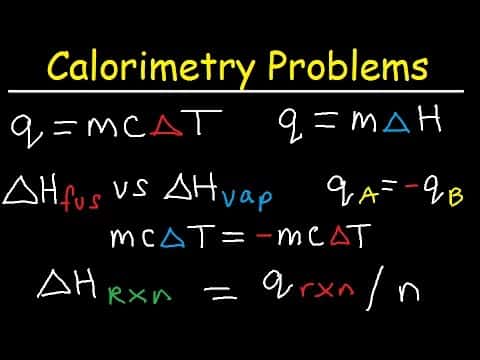
Calorimetry
The complying with< img align=" absmiddle" boundary =" 0" elevation ![]() =” 38 “src=” https://mmsphyschem.com/wp-content/uploads/2017/05/thermotag-2258852.gif” size=” 231″ >
=” 38 “src=” https://mmsphyschem.com/wp-content/uploads/2017/05/thermotag-2258852.gif” size=” 231″ >
might serve.
1) Determine the enthalpy modification for the burning of ethane to generate co2 and also water vapor.
2C2H6( g) + 7O2( g)![]() < img elevation=" 12" src=“https://mmsphyschem.com/wp-content/uploads/2017/05/arrow-6935237.gif” size=” 43″ >
< img elevation=" 12" src=“https://mmsphyschem.com/wp-content/uploads/2017/05/arrow-6935237.gif” size=” 43″ >
4CO 2( g) +6H 2 O( g)(
a) -551 kJ
( b) -2856 kJ
( c) -3120 kJ
(
d ) 551 kJ( e) 2856 kJ 2 )1.00 g ofstearic acid,( CH 3(CH2) 16 CARBON MONOXIDE 2 H), was melted in a bomb calorimeter. The bomb had a warm ability of 652 J/ ºC as well as a 500. g water tank. If the temperature level climbed from 25.0 to 39.3 ºC, just how much warmth was launched when the stearic acid was melted?( a) 39.2 kJ
( b) 29.9 kJ
( c) 108 kJ
( d) 9.32 kJ
3) Determine
the enthalpy adjustment for the burning of butane to create co2 as well as water vapor. 2C 4 H 10 (g )+ 13O 2 (g )< img elevation=" 12 "src=" https://mmsphyschem.com/wp-content/uploads/2017/05/arrow-6935237.gif" size=" 43" > 8CO 2( g) + 10H2O( g )( a) 3790 kJ( b) 253 kJ( c) -253 kJ( d) -5314 kJ( e) -4230 kJ 4) 1.00 g of sucrose( table sugar ), C 12 H 22 O 11, was melted in abomb calorimeter. The bomb
had a warmth ability of 547
J
/ ºC and also a 775
g water tank. If the temperature level increased from 25.00 to 29.35 ºC, just how much warm
was launched when the sugar was shed ?( a) 111 kJ (b) 14.1 kJ( c) 16.5 kJ( d) 2.38 kJ 5)Determine the enthalpy adjustment for the burning of acetylene to generate co2 as well as water vapor. 2C 2 H 2 (g)+ 5O 2( g)< img elevation =" 12" src =" https://mmsphyschem.com/wp-content/uploads/2017/05/arrow-6935237.gif"
size=” 43
” > 4CO 2( g )+
2H 2 O( g) (a
) 2511 kJ
(
b ) 862 kJ( c) -2511 kJ( d) -862 kJ( e )-2599 kJ 6) 1.00 g of alanine, CH 3 CH (NH 3) CARBON MONOXIDE 2 H, was melted in a bomb calorimeter
. The calorimeterhad a warm capability of 719J/ ºC as well as a 450. g water tank. If the temperature level increased from 20.00 to 26.92 ºC, just how much warmth was launched when the alanine was melted?( a)
4.98 kJ
( b) 18.0 kJ
( c) 70.0 kJ
( d) 13.0 kJ
7) Determine the enthalpy
adjustment
for the burning of ammonia tocreate nitrogen monoxide and also water vapor. 4NH 3 (g) +5O 2( g )< img elevation=" 12" src=" https://mmsphyschem.com/wp-content/uploads/2017/05/arrow-6935237.gif "size=" 43 "> 4NO (g)+ 6H 2 O( g) (a) -105 kJ (b) -1170 kJ( c) -905 kJ( d )105 kJ( e )905 kJ. 8) 1.00 g of high levels of caffeine
, C 8 H 10 O 2 N 4, was shed ina bomb calorimeter. The calorimeter had a warmth ability of 563 J
/ ºC and also a 692
g
water storage tank. If the temperature level climbed from 20.00 to 26.32 ºC, just how much warm was launched when the high levels of caffeine was melted?( a) 91.0 kJ( b)
3.56 kJ( c) 18.3kJ( d)![]() 21.9 kJ. 9)Determine the enthalpy modification for the burning of dihydrogen sulfide to create sulfur dioxide
21.9 kJ. 9)Determine the enthalpy modification for the burning of dihydrogen sulfide to create sulfur dioxide
as well as water vapor. 2H 2
S( g )+ 3O 2(
g)< img elevation=
” 12 “src =”
https://mmsphyschem.com/wp-content/uploads/2017/05/arrow-6935237.gif“size=” 43″ > 2SO 2(g) +2H 2 O( g)( a )-1124 kJ( b) -1036 kJ (c) -518 kJ( d) 1036 kJ( e) 518 kJ. 10 )Determine the enthalpy modification for the burning of fluid methanol to generate co2 as well as water vapor. 2CH 3 OH( l)+ 3O 2( g)< img elevation=
” 12 “src =” https://mmsphyschem.com/wp-content/uploads/2017/05/arrow-6935237.gif ” size=” 43
” > 2CO 2( g)+ 4H 2 O( g )( a) -1277 kJ( b) -1453 kJ (c) -397 kJ( d) 1277 kJ( e )397 kJ Residence Online Assignments