Acid-Base Titrations (Second Derivative)
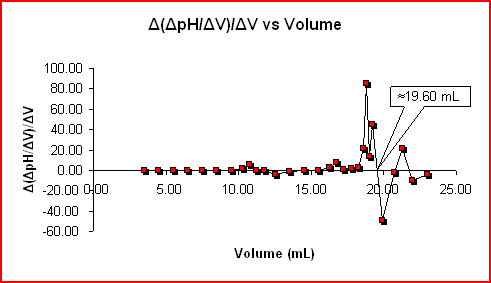
< img align=" right" elevation =" 233" src =" https://mmsphyschem.com/wp-content/uploads/2019/11/titr-8057930.jpg" size =" 124 "> The adhering to information worths are based upon the example information showed on the Acid-Base Titrations (Titration Curve )web page. The information is made use of to chart Δ( ΔpH/ ΔV)/ ΔV vs Volume which stands for the 2nd by-product of the pH information (Δ( ΔpH/ ΔV)/ ΔV).
Vave (mL) ΔpH/ ΔV ΔV( mL) Δ( ΔpH) V ave( mL) Δ (ΔpH/ ΔV)/ ΔV V ave( mL) ΔpH/ ΔV ΔV (mL) Δ( ΔpH) V ave( mL) Δ (ΔpH/ ΔV)/ ΔV 1.50 0 01.00 0 0 0 15.50 4.90. 1.00. 0.15. 15.00. 0.15. 2.50 3.24. 1.00. 0 0 0 16.25. 6.93. 0.75. 2.03. 15.88. 2.71. 3.50 3.39. 1.00. 0.15. 3.00 0.15. 16.75. 10.80. 0.50. 3.87. 16.50. 7.73. 4.50 3.54. 1.00 . 0.40. 17.00. 0.80. 5.50 3.63. 1.00 5.00 0.09. 17.75. 11.90. 0.50. 0.70. 17.50. 1.40. 6.50 3.78. 1.00. 0.15. 6.00 0.15. 18.25. 13.20 . 7.00 0.07 . 18.43. 21.88. 8.50 3.98. 1.00 . 0.134.11. 1.00. 0.13. 9.00 0.13. 19.00. 40.75. 0.20 . 10.25 5.60. 0.75. 1.49. 9.88 1.99. 19.20. 49.75. 0.20 . -34.75. . 0.10. 11.00 0.20. 20.75. 12.82 . 0.85. 10.78. 21.00. 21.55– 3.76 23.00. 12.30. 1.00.- 3.97.4.75. 1.00. 0.15. 14.00 0.15. 0 0 0 0 0 0 The equivalence factor represents thequantitylocated on the Acid-Base Titrations( Titration Curve) web page remains in close contract with the 19.60 mL.
This isexamine out Acid-Base Titrations( Titration Curve), Acid-Base
authorization
or mechanical and also access , expenses